|
Atoms, Elements and Compounds
- All substances are made of atoms. An atom is the smallest part of an element that can exist
- Atoms of each element are represented by a chemical symbol, e.g. O represents an atom of oxygen, Na represents an atom of sodium
- There are about 100 different elements. Elements are shown in the periodic table
- Compounds are formed from elements by chemical reactions
- Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change
- Compounds contain two or more elements chemically combined in fixed proportions and can be represented by formulae using the symbols of the atoms from which they were formed
- Compounds can only be separated into elements by chemical reactions
- Chemical reactions can be represented by word equations or equations using symbols and formulae
Atomic Structure
- Atoms are very small, having a radius of about 1x10-10 metres
- The basic structure of an atom is a positively charged nucleus composed of both protons and neutrons surrounded by negatively charged electrons
- The radius of a nucleus is less than 1/10 000 of the radius of an atom
- Most of the mass of an atom is concentrated in the nucleus
- In the periodic table, each element appears with two numbers: the atomic number and the mass number
- The atomic number of an element is equal to the number of protons in the nucleus of an atom of that element
- In a neutral atom (an atom with no overall charge), the number of protons (+) is equal to the number electrons (-)
- Different elements have different atomic numbers, and therefore different numbers of protons in the nucleus of their atom
- The mass number of an element is equal to the number of neutrons + protons in the nucleus of an atom of that element
- To determine the number of neutrons in the nucleus of an atom of an element, the atomic number should be subtracted from the mass number
Electronic Configuration
- The electrons are arranged at different distances from the nucleus (in different energy levels, or shells)
- The electron arrangements may change with the absorption of electromagnetic radiation (move further from the nucleus; a higher energy level) or by the emission of electromagnetic radiation (move closer to the nucleus; a lower energy level)
- The electrons in an atom occupy the lowest available energy levels first (which are the innermost available shells)
- Electrons will fill the lowest available energy level before moving to a higher energy level
- The electron shell (or energy level) closest to the nucleus can take two electrons
- The second energy level takes up to eight electrons
- If the outermost energy level is full, the atom will be stable (unreactive)
- The electronic structure of an atom can be represented by numbers or by a Bohr diagram. For example, the electronic structure of sodium is 2,8,1 or showing two electrons in the lowest energy level, eight in the second energy level and one in the third energy level
Isotopes
- Atoms of the same element can have different numbers of neutrons. These atoms are called isotopes of that element
- Isotopes are atoms of the same element with different mass numbers due to different numbers of neutrons in the nucleus
- Isotopes of an element will all have the same atomic number, because the number of protons is always the same for all atoms of that element
- Isotopes of an element will have different mass numbers because they have different numbers of neutrons
- Isotopes of an element can be represented as ‘[element name]-[mass number]’, for e.g., carbon-12 and carbon-14
- The relative atomic mass of an element is an average value that takes account of the abundance of the isotopes of the element
- The relative atomic mass does not have a unit
The Atomic Model
- New experimental evidence may lead to a scientific model being changed or replaced
- Before the discovery of the electron, atoms were thought to be tiny spheres that could not be divided
- JJ Thompson discovered the electron
- JJ Thompson developed the plum pudding model of the atom. This model suggested that the atom is a ball of positive charge with negative electrons embedded in it
- Geiger, Marsden and Rutherford carried out a famous experiment called the Gold Foil (or alpha scattering) experiment. The results from this experiment led to the conclusion that the mass of an atom was concentrated at the centre (nucleus) and that the nucleus was charged
- The nuclear model of the atom replaced the plum pudding model and is the model we use to this day
- Niels Bohr adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observation
- James Chadwick discovered the neutron
- Our knowledge of atoms is still developing today
The Periodic Table
- The elements in the periodic table are arranged in order of atomic number and so that elements with similar properties are in columns, known as groups
- The table is called a periodic table because similar properties occur at regular intervals (periodically)
- Elements in the same group in the periodic table have the same number of electrons in their outer shell (outer electrons) and this gives them similar chemical properties
- The early periodic tables were incomplete, and some elements were placed in inappropriate groups if the strict order of atomic weights was followed
- Mendeleev overcame some of the problems by leaving gaps for elements that he thought had not been discovered and, in some places, changed the order based on atomic weights
- The elements in Group 0 of the periodic table are called the noble gases
- They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons
- A stable electronic configuration is one in which the outer shell of electrons is full
- The noble gases have eight electrons in their outer shell, except for helium, which has only two electrons
- The boiling points of the noble gases increase with increasing relative atomic mass (going down the group)
- The elements in Group 1 of the periodic table are known as the alkali metals
- The Alkali metals have one electron in their outer shell
- Alkali metals are soft, shiny metals with low melting and boiling points
- In Group 1, the reactivity of the elements increases going down the group
- Alkali metals react vigorously with water to form the metal hydroxide and hydrogen gas
- The equations for the alkali metals’ reactions with water take the form ‘lithium + water →
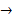 lithium hydroxide + hydrogen’ which can also be written as ‘2Li + 2H2O → lithium hydroxide + hydrogen’ which can also be written as ‘2Li + 2H2O →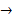 2LiOH + H2’. 2LiOH + H2’.
- The metal oxides produced by the reactions of alkali metals with water are alkaline
- When added to water, the alkali metals will move rapidly across the surface of the water. This is caused by the production of hydrogen gas.
- When reacting with water, the alkali metals will form a spherical shape. This is caused by the heat of the reaction.
- When alkali metals react with water, sometimes the heat of the reaction will cause the hydrogen gas produced to be set alight. This causes an orange flame to be seen (in the case of sodium) or a lilac flame to be seen (in the case of potassium).
- The alkali metals react with oxygen to form metal oxides
- The alkali metals are stored under oil to prevent reaction with oxygen, also known as corrosion.
- Corrosion makes the alkali metals less shiny.
- The equations for the alkali metals reactions with oxygen take the form ‘lithium + oxygen →
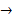 lithium oxide’ which can also be written as ‘4Li + O2 → lithium oxide’ which can also be written as ‘4Li + O2 →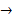 2Li2O’ 2Li2O’
- The elements in Group 7 of the periodic table are known as the halogens
- The halogens all have similar reactions because they all have seven electrons in their outer shell.
- The halogens are non-metals and consist of diatomic molecules
- Diatomic molecules consist of pairs of atoms bonded together
- Chlorine is a pale green gas at room temperature
- Bromine is a brown/orange liquid at room temperature
- Iodine is a black or purple solid at room temperature
- In Group 7, the further down the group an element is the higher its relative molecular mass, melting point and boiling point
- The melting and boiling points of the halogens increase going down the group because the molecules become larger, the forces between the molecules become stronger, and so more energy is needed to overcome these forces.
- In Group 7, the reactivity of the elements decreases going down the group. This is because the atomic radius increases moving down the group. The further the outer shell is from the nucleus, the harder it will be to attract the electron required for a stable electronic configuration.
- The halogens react with metals to produce metal halides, for e.g., sodium + chlorine →
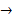 sodium chloride sodium chloride
- The halogens react with hydrogen to produce hydrogen halides, for e.g., hydrogen + chlorine →
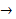 hydrogen chloride hydrogen chloride
- A more reactive halogen can displace a less reactive halogen from an aqueous solution of its salt
- The transition elements are metals including Cr, Mn, Fe, Co, Ni, Cu with similar properties which are different from those of the elements in Group 1
- Many transition elements have ions with different charges form coloured compounds and are useful as catalysts
|
Disciplinary Knowledge
- Recognise and use expressions in standard form
- Use prefixes and powers of ten for orders of magnitude (e.g. tera, giga, mega, kilo, centi, milli, micro and nano)
- Make order of magnitude calculations
- Recognise that scientific methods and theories change over time
- Critique and evaluate models, including:
- Make predictions or calculate quantities based on the model or show its limitations
- Give examples of ways in which a model can be tested by observation or experiment
- Evaluate the strengths and limitations of a model
- Interpret a line (scatter) graph
- Understand and use the symbols: =, <>, >, ∝, ~
Practical Skills
|











